About immunotherapy
Immunotherapy What is immunotherapy?
Immunotherapy is also sometimes called biologic therapy or biotherapy. It is treatment that uses certain parts of the immune system to fight diseases such as cancer. This can be done in a couple of ways:
- Stimulating your own immune system to work harder or smarter to attack cancer cells
- Giving you immune system components, such as man-made immune system proteins For a long time doctors suspected that the immune system had an effect on certain cancers. Even before the immune system was well understood, William Coley, MD, a New York surgeon, first noted that getting an infection after surgery seemed to help some cancer patients. In the late 1800s, he began treating cancer patients by infecting them with certain kinds of bacteria, which came to be known as Coley toxins. Although he had some success, his technique was overshadowed when other forms of cancer treatment, such as radiation therapy, came into use. Since then, doctors have learned a great deal about the immune system. This has led to research into how it can be used to treat cancer, using many different approaches. In the last few decades immunotherapy has become an important part of treating several types of cancer.
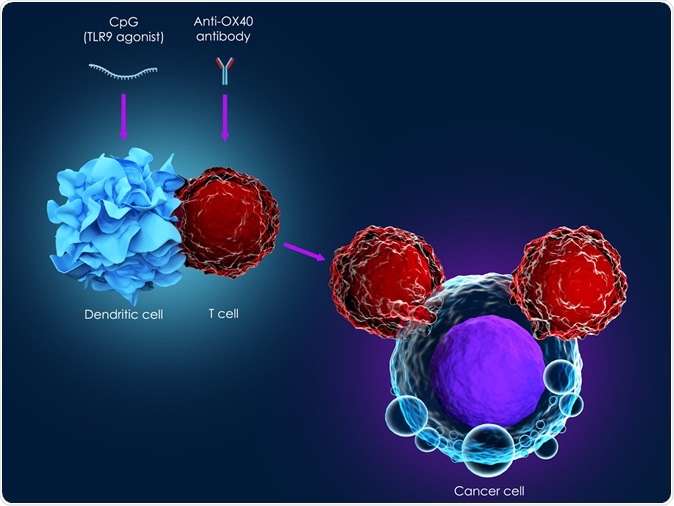
As researchers have learned more about the body’s immune system in recent years, they have begun to figure out how it might be used to treat cancer more effectively. Newer treatments now being tested seem to work better and will have a greater impact on the outlook for people with cancer in the future.
What the immune system does
Your immune system is a collection of organs, special cells, and substances that help protect you from infections and some other diseases. Immune system cells and the substances they make travel through your body to protect it from germs that cause infections. They also help protect you from cancer in some ways.
It may help to think of your body as a castle. Think of viruses, bacteria, and parasites as hostile, foreign armies that are not normally found in your body. They try to invade your body to use its resources to serve their own purposes, and they can hurt you in the process. In fact, doctors often use the word foreign to describe invading germs or other substances not normally found in the body. The immune system is your body’s defense force. It helps keep invading germs out, or helps kill them if they do get into your body.
The immune system basically works by keeping track of all of the substances normally found in the body. Any new substance in the body that the immune system does not recognize raises an alarm, causing the immune system to attack it. Substances that cause an immune system response are called antigens. The immune response can lead to destruction of anything containing the antigen, such as germs or cancer cells.
Germs such as viruses, bacteria, and parasites have substances on their outer surfaces, such as certain proteins, that are not normally found in the human body. The immune system sees these foreign substances as antigens. Cancer cells are also different from normal cells in the body. They often have unusual substances on their outer surfaces that can act as antigens. But the immune system is much better at recognizing and attacking germs than cancer cells. Germs are very different from normal human cells and are often easily seen as foreign, but cancer cells and normal cells have fewer clear differences. Because of this the immune system may not always recognize cancer cells as foreign. Cancer cells are less like soldiers of an invading army and more like traitors within the ranks of the human cell population.
Clearly the immune system’s normal ability to fight cancer is limited, because many people with healthy immune systems still develop cancer. The immune system may not see the cancer cells as foreign because the cancer cells (and their antigens) are not different enough from those of normal cells. Sometimes the immune system recognizes the cancer cells, but the response may not be strong enough to destroy the cancer. Cancer cells themselves may also give off substances that keep the immune system in check.
To overcome this, researchers have designed ways to help the immune system recognize cancer cells and strengthen its response so that it will destroy them.
Types of immunotherapy
There are many types of cancer treatments that could be thought of as immunotherapy. Some work by stimulating your body’s own immune system to fight the disease. This may be done by boosting the immune system in a very general way, or by training the immune system to attack some part of cancer cells specifically. Other treatments sometimes considered immunotherapy use immune system components (such as proteins called antibodies) that are made in the lab. Some of these boost the immune system once they are in the body. Others don’t really affect the immune system much, if at all. Instead, the antibodies themselves target specific parts of cancer cells, stopping them from growing or making them die. The main types of immunotherapy now being used to treat cancer are discussed in the following sections. They include:
- Monoclonal antibodies: These are man-made versions of immune system proteins. Antibodies can be very useful in treating cancer because they can be designed to attack a very specific part of a cancer cell.
- Cancer vaccines: Vaccines are substances put into the body to start an immune response against certain diseases. We usually think of them as being given to healthy people to help prevent infections. But some vaccines may help prevent or treat cancer.
- Non-specific immunotherapies: These treatments boost the immune system in a very general way, but this may still result in more activity against cancer cells. Immunotherapy drugs are now used to treat a number of cancers, including cancers of the bladder, breast, colon, kidney, lung, and prostate, as well as leukemia, lymphoma, multiple myeloma, and melanoma. If you would like more information about immunotherapy as a treatment for a specific cancer, please see our detailed guide for that cancer. Many other types of immunotherapy are now being studied for use against cancer. Some of these are discussed in the section “What’s new in immunotherapy research?”
Many types of immunotherapy work by targeting specific parts of cancer cells. As such, they can be thought of as a form of targeted therapy, which differs from less specific treatments like chemotherapy. But there are also other targeted treatments that zero in on parts of cancer cells that are not immunotherapies. For more information on targeted drugs, see our document, Targeted Therapy.
Monoclonal antibodies
One way the immune system normally attacks foreign substances in the body is by making large numbers of different antibodies. An antibody is a “sticky” protein that targets a specific antigen. Antibodies circulate in the body until they find and attach to the antigen. Once attached, they recruit other parts of the immune system to destroy the cells containing the antigen. Many copies of a specific antibody can be made in the lab. These are known as monoclonal antibodies (mAbs or moAbs). These antibodies can be useful in fighting diseases because they can be designed specifically to only target a certain antigen, such as one that is found on cancer cells. Monoclonal antibodies are now used to treat many diseases, including some types of cancer. A major advantage of these drugs is that because they are so specific, they may have only mild side effects, unlike some other cancer treatments. But researchers first have to identify the right antigen to attack. For cancer, this is not always easy, and so far mAbs have proven to be more useful against some cancers than others. Over the past 15 years or so, the US Food and Drug Administration (FDA) has approved about a dozen mAbs to treat certain cancers. As researchers have found more antigens that are linked to cancer, they have been able to make monoclonal antibodies against more and more cancers. Clinical trials of newer mAbs are now being done on many types of cancer.
Types of monoclonal antibodies
Two types of monoclonal antibodies are used in cancer treatments:
- Naked mAbs are antibodies that work by themselves. There is no drug or radioactive material attached to them. These are the most commonly used mAbs at this time.
- Conjugated mAbs are those joined to a chemotherapy drug, radioactive particle, or a toxin (a substance that poisons cells). These mAbs work, at least in part, by acting as homing devices to take these substances directly to the cancer cells.
Naked monoclonal antibodies
Most naked mAbs attach to antigens on cancer cells, but some work by binding to antigens on other, non-cancerous cells, or to even free-floating proteins. Naked mAbs can work in different ways. Some may boost a person’s immune response against cancer cells. Others work by blocking specific proteins that help cancer cells grow. (Some may do both.)
Some naked MAbs attach to cancer cells to act as a marker for the body’s immune system to destroy them. An example of this is alemtuzumab (Campath®), which is used to treat some patients with chronic lymphocytic leukemia. Alemtuzumab is an antibody that binds to the CD52 antigen, which is found on immune cells called B cells and T cells. Once attached, the antibody triggers the destruction of the cell by the immune system. Some naked mAbs work mainly by attaching to and blocking specific antigens that are important signals for cancer cells (or other cells that help cancer cells grow or spread).
For example, trastuzumab (Herceptin®) is an antibody against the HER2/neu protein. A large amount of this protein is present on the cells in some types of cancer. When becoming active. It is used to treat breast and stomach cancers that have large amounts of this protein.
Conjugated monoclonal antibodies
Monoclonal antibodies attached to a radioactive substance, drug, or toxin, are called conjugated mAbs. The mAb is used as a homing device to take one of these substances directly to the cancer cells. The mAb circulates in the body until it can find and hook onto the target antigen. It then delivers the toxic substance where it is needed most. This lessens the damage to normal cells in other parts of the body.
Conjugated mAbs are also sometimes referred to as tagged, labeled, or loaded antibodies.
They can be divided into groups depending on what they are linked to.
- mAbs with radioactive particles attached are referred to as radiolabeled, and treatment with this type of antibody is known as radioimmunotherapy (RIT).
- mAbs with chemotherapy drugs attached are referred to as chemolabeled.
- mAbs attached to cell toxins are called immunotoxins.
Radiolabeled antibodies: Radio labeled antibodies have small radioactive particles attached to them. Ibritumomab tiuxetan (Zevalin®) and tositumomab (Bexxar®) are examples of radiolabeled mAbs. Both of these are antibodies against the CD20 antigen, but they each have a different radioactive particle attached. They deliver radioactivity directly to cancerous B cells and can be used to treat some types of non-Hodgkin lymphoma.
Chemolabeled antibodies: These mAbs have powerful chemotherapy drugs attached to them. (The chemotherapy drug is often too powerful to be used on its own – it would cause too many side effects if not attached to an mAb.)
The only chemolabeled antibody approved by the FDA to treat cancer at this time is brentuximab vedotin (Adcetris). This drug is made up of an antibody that targets the CD30 antigen (found on B cells and T cells), attached to a chemo drug called MMAE. It is used to treat Hodgkin lymphoma and anaplastic large cell lymphoma that is no longer responding to other treatments. Immunotoxins: These mAbs have cell poisons (toxins) attached to them, which makes them similar in many ways to chemolabeled mAbs. At this time no immunotoxins are approved to treat cancer, although many are being studied.
However, a related drug known as denileukin diftitox (Ontak®) is being used to treat some cancers. It consists of an immune system protein known as interleukin-2 (IL-2) attached to a toxin from the germ that causes diphtheria. Although it’s not an mAb, IL-2 normally attaches to certain cells in the body that contain the CD25 antigen, which makes it useful for delivering the toxin to these cells. Denileukin diftitox is used to treat lymphoma of the skin (also known as cutaneous T-cell lymphoma). It is also being studied to be used against a number of other cancers.
Possible side effects of monoclonal antibodies
Monoclonal antibodies are given intravenously (injected into a vein). Compared with the side effects of chemotherapy, the side effects of naked mAbs are usually fairly mild and are often more like an allergic reaction. These are more common while the drug is first being given. Possible side effects can include:
- Fever
- Chills
- Weakness
- Headache
- Nausea
- Vomiting
- Diarrhea
- Low blood pressure
- Rashes
Some mAbs can also have other side effects that are related to the antigens they target. For example, bevacizumab (Avastin®), an mAb that targets tumor blood vessel growth, can cause side effects such as high blood pressure, bleeding, poor wound healing, blood clots, and kidney damage. Conjugated antibodies may pack more of a punch than naked mAbs, but they often cause more side effects. The side effects depend on which type of substance they’re attached to.
Cancer vaccines
Most of us know about vaccines given to healthy people to help prevent infections, such as measles and mumps. These vaccines use weakened or killed germs like viruses or bacteria to start an immune response in the body. Getting the immune system ready to defend against these germs helps keep people from getting infections. Most cancer vaccines work the same way, but they usually prime the immune system to attack cancer cells in the body. The goal is to help treat cancer or to help prevent it from coming back after other treatments. But there are some vaccines that may actually help prevent certain cancers.
Vaccines to help prevent cancer
Many people might not realize it, but some cancers are caused by viruses. Vaccines that help protect against infections with these viruses might also help prevent some of these cancers. Some strains of the human papilloma virus (HPV) have been linked to cervical, anal, throat, and some other cancers. Vaccines against HPV may help protect against some of these cancers. People who have chronic (long-term) infections with the hepatitis B virus (HBV) are at higher risk for liver cancer. Getting the HBV vaccine to help prevent this infection may therefore lower some people’s risk of getting liver cancer.
These are traditional vaccines in that they target the viruses that can cause certain cancers. They may help protect against some cancers, but they don’t target cancer cells directly. These types of vaccines are only useful for cancers known to be caused by infections.
But most cancers, such as colorectal, lung, prostate, and breast cancers, are not thought to be caused by infections. Doctors are not yet sure if it will be possible to make vaccines to prevent these other cancers. Some researchers are now trying, but this research is still in very early stages. Even if such vaccines prove to be possible, it will be many years before they become available.
Vaccines to help treat cancer
Cancer treatment vaccines are different from the vaccines that work against viruses. These vaccines try to get the immune system to mount an attack against cancer cells in the body. Instead of preventing disease, they are meant to get the immune system to attack a disease that already exists.
A cancer treatment vaccine uses cancer cells, parts of cells, or pure antigens to increase the immune response against cancer cells that are already in the body. Vaccines are often combined with other substances or cells called adjuvants that help boost the immune response even further.
Cancer vaccines don’t just boost the immune system in general; they cause the immune system to attack cells with one or more specific antigens. And because the immune system has special cells for memory, it’s hoped that the drugs will help keep cancer from coming back.
Sipuleucel-T (Provenge®)
At this time, this is the only vaccine approved by the US Food and Drug Administration (FDA) to help treat cancer. It is used to treat advanced prostate cancer that is no longer being helped by hormone therapy.
For this vaccine, immune system cells are removed from the patient’s blood and sent to a lab. There they are exposed to chemicals that turn them into special cells called dendritic cells. They are also exposed to a protein called prostatic acid phosphatase (PAP), which should produce an immune response against prostate cancer.
The dendritic cells are then given back to the patient by infusion into a vein (IV). This process is repeated twice more, 2 weeks apart, so that the patient gets 3 doses of cells. Back in the body, the cells help other immune system cells attack the patient’s prostate cancer. Although the vaccine does not cure prostate cancer, it has been shown to help extend patient’s lives by several months on average. Studies to see if this vaccine can help men with less advanced prostate cancer are now being done. Side effects are usually mild and can include fever, chills, fatigue, back and joint pain, nausea, and headache. A few men may have more severe symptoms, including problems breathing and high blood pressure. Other cancer vaccines have shown some promise in clinical trials, but have yet to be approved in the United States to treat cancer. Some of these are described in the section, “What’s new in immunotherapy research?”
Types of cancer treatment vaccines being studied
Several types of cancer vaccines are now being studied, with a few reaching late stage clinical trials.
Tumor cell vaccines: These vaccines are made from actual cancer cells that have been removed during surgery. The cells are treated in the lab, usually with radiation, so they cannot form more tumors. In most cases, doctors also change the cells in certain ways, often by adding chemicals or new genes, to make them more likely to be seen as foreign by the immune system. The cells are then injected into the patient. The immune system recognizes antigens on these cells, then seeks out and attacks any other cells with these antigens that are still in the body.
Most tumor cell vaccines are autologous, meaning the vaccine is made from killed tumor cells taken from the same person in whom they will later be used. In other words, cells
are injected back into you. Some vaccines are allogeneic, meaning the cells for the vaccine come from someone other than the patient being treated. Allogeneic vaccines areeasier to make than autologous vaccines, but it is not yet clear if one type is more effective than the other.
Antigen vaccines: These vaccines boost the immune system by using only one antigen (or a few), rather than whole tumor cells that contain many thousands of antigens. The antigens are usually proteins or pieces of proteins called peptides.
Antigen vaccines may be specific for a certain type of cancer, but they are not made for a specific patient like autologous cell vaccines are. Scientists often combine several antigens in a vaccine to try to get a stronger immune response.
Dendritic cell vaccines: Dendritic cells are special immune cells in the body that help the immune system recognize cancer cells. They break down cancer cells into smaller pieces (including antigens), then hold out these antigens so other immune cells called T cells can see them. This makes it easier for the immune system cells to recognize and attack cancer cells.
Dendritic cell vaccines are autologous vaccines (made from the person in whom they will be used), and must be made individually for each patient. The process used to create them is complex and expensive. Doctors remove some immune cells from the blood and expose them in the lab to cancer cells or cancer antigens, as well as to other chemicals that turn them into dendritic cells and help them grow. The dendritic cells are then injected back into the patient, where they should provoke an immune response to cancer cells in the body.
Sipuleucel-T (Provenge), which is used to treat advanced prostate cancer, is an example of a dendritic cell vaccine.
DNA vaccines: When tumor cells or antigens are injected into the body as a vaccine, they may cause the desired immune response at first, but they may become less effective over time. This is because the immune system recognizes them as foreign and quickly destroys them. Without any further stimulation, the immune system often returns to its normal (pre-vaccine) state of activity. To get around this, scientists have looked for a way to provide a steady supply of antigens to keep the immune response going. DNA is the substance in cells that contains the genetic code for the proteins that cells make. Vectors (see next section) can be given bits of DNA that code for protein antigens. When the vectors are then injected into the body, this DNA might be taken up by cells and can instruct them to make specific antigens, which would then provoke the desired immune response. These types of therapies are called DNA vaccines.
Vector-based vaccines: These vaccines use special delivery systems (called vectors) to make them more effective. They aren’t really a separate category of vaccine; for example, there are vector-based antigen vaccines and vector-based DNA vaccines. Vectors are special viruses, bacteria, yeast cells, or other structures that can be used to get antigens or DNA into the body. The vectors are often germs that have been altered to make sure they can no longer cause disease.
Vectors may be helpful in making vaccines for a number of reasons. First, they may be used to deliver more than one cancer antigen at a time, which may make the body’s immune system more likely to mount a response. Second, vectors such as viruses and bacteria may trigger their own immune responses from the body, which may help make the overall immune response even stronger. Finally, these vaccines may be easier and less expensive to make than some other vaccines.
Non-specific immunotherapies and adjuvants
Non-specific immunotherapies do not target a certain cell or antigen. They stimulate the immune system in a very general way, but this may still result in more activity against cancer cells.
Some non-specific immunotherapies can be given by themselves as cancer treatments. Others are used as adjuvants (along with a main treatment) to boost the immune system to improve how well another type of immunotherapy (such as a vaccine) works. Some are used by themselves against some cancers and as adjuvants against others.
Cytokines
Cytokines (pronounced SY-toh-kines) are chemicals made by immune system cells. They are crucial in controlling the growth and activity of other immune system cells and blood cells in the body.
Man-made versions of some cytokines can be given alone to boost the immune system, or they can be given along with tumor vaccines as adjuvants. Some man-made cytokines are used to lessen the side effects of other treatments such as chemotherapy. They can help the bone marrow make more white blood cells, red blood cells, or platelets when their levels in the body have gotten too low. While this is important in cancer treatment, it isn’t truly immunotherapy. Cytokines are injected, either under the skin, into a muscle, or into a vein. The most common ones are discussed here.
Interleukins
Interleukins are a group of cytokines that act as chemical signals between white blood cells.
Interleukin-2 (IL-2) helps immune system cells grow and divide more quickly. When a man-made version of IL-2 was approved by the US Food and Drug Administration in 1992 to treat advanced kidney cancer, it became the first true immunotherapy approved to be used alone in treating cancer. Since that time, it has also been approved to treat people with metastatic melanoma.
IL-2 can be used as a single drug treatment for these cancers, or it may be combined with chemotherapy or with other cytokines such as interferon-alfa. (It is also being studied for use as an adjuvant along with some vaccines.) Using IL-2 with these treatments might help make them more effective against some cancers, but the side effects of the combined treatment are also increased.
Side effects of IL-2 may include flu-like symptoms such as chills, fever, fatigue, and confusion. Most people gain weight. Some have nausea, vomiting, or diarrhea. Many people develop low blood pressure, which can be treated with other medicines. Rare but potentially serious side effects include an abnormal heartbeat, chest pain, and other heart problems. Because of these potential side effects, if IL-2 is given in high doses, it must be done in the hospital (as an inpatient). Other interleukins, such as IL-7, IL-12, and IL-21, are now being studied for use against cancer too, both as adjuvants and as stand-alone agents.
Interferons
These cytokines, first discovered in the late 1950s, help the body resist virus infections and cancers. The types of interferon (IFN) are named after the first 3 letters of the Greek alphabet: IFN-alfa, IFN-beta, and IFN-gamma. Only IFN-alfa is used to treat cancer. It boosts the ability of certain immune cells to attack cancer cells. It may also slow the growth of cancer cells directly, as well as the blood vessels that tumors need to grow.
The FDA has approved IFN-alfa for use against these cancers:
- Hairy cell leukemia
- Chronic myelogenous leukemia
- Follicular non-Hodgkin lymphoma
- Cutaneous (skin) T-cell lymphoma
- Kidney cancer
- Melanoma
- Kaposi sarcoma
Side effects of interferons may include flu-like symptoms (chills, fever, headache, fatigue, loss of appetite, nausea, vomiting), low white blood cell counts (which increase the risk of infection), skin rashes, and thinning hair. These side effects can be severe and can make treatment with interferon hard to tolerate for many people. Most side effects do not last long after the treatment stops, but fatigue can last longer. Other rare long-term effects include damage to nerves, including those in the brain and spinal cord.
Granulocyte-macrophage colony-stimulating factor
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that causes the bone marrow to make more of certain types of immune system cells and blood cells. A man-made version (known as sargramostim or Leukine®) is often used to boost white blood cell counts after chemotherapy.
GM-CSF is also being tested against cancer as a non-specific immunotherapy and as an adjuvant given with other types of immunotherapies. Clinical trials of GM-CSF, alone or with other immunotherapies, are being done in people with many different types of cancer.
Common side effects of GM-CSF include flu-like symptoms (fever, headaches, muscle aches), rashes, facial flushing, and bone pain.
Other drugs that boost the immune system
Some other drugs boost the immune system in a non-specific way, similar to cytokines. But unlike cytokines, these drugs are not naturally found in the body.
Thalidomide
Thalidomide (Thalomid®) is used as a treatment for multiple myeloma and some other cancers. It is thought to work in a general way by boosting the immune system, although it’s not exactly clear how it does this.
Side effects of thalidomide can include drowsiness, fatigue, severe constipation, and neuropathy (painful nerve damage). The neuropathy can be severe, and may not go away after the drug is stopped. There is also an increased risk of serious blood clots (that start in the leg and can travel to the lungs). Because thalidomide causes severe birth defects if taken during pregnancy, this drug can only be obtained through a special program run by the drug company that makes it.
Lenalidomide
Lenalidomide (Revlimid®) is a newer drug that is similar to thalidomide. It is used to treat multiple myeloma and some other cancers. The most common side effects of lenalidomide are low platelet and low white blood cell counts. It can also cause painful nerve damage. The risk of blood clots is not as great as that seen with thalidomide, but it is still increased. Like thalidomide, access to lenalidomide is tightly controlled out of concern about possible serious birth defects.
Bacille Calmette-Guérin
Bacille Calmette-Guérin (BCG) is a germ related to the one that causes tuberculosis. Unlike its bacterial “cousin,” BCG does not cause serious disease in humans, but it does infect human tissues and helps activate the immune system. This makes BCG useful as a form of cancer immunotherapy. BCG was one of the earliest immunotherapies used against cancer and is still being used today. BCG is FDA-approved for early stage bladder cancer. It is placed directly into the bladder through a catheter. The body’s immune system cells are attracted to the bladder and activated by BCG, which in turn affects the bladder cancer cells. Treatment with BCG may cause symptoms that are like having the flu, such as fever, chills, and fatigue. It can also cause a burning feeling in the bladder. BCG may also be used to treat some melanoma skin cancers by injecting it directly into the tumors.
Imiquimod
Imiquimod (Aldara®) is a drug that, when applied as a cream, stimulates a local immune response against skin cancer cells. It is used to treat some very early stage skin cancers (or pre-cancers), especially if they are on sensitive areas such as on the face. The cream is applied anywhere from once a day to twice a week for several months. Some people may have serious skin reactions to this drug.
What’s new in immunotherapy research?
Immunotherapy is a very active area of cancer research. Many scientists and doctors around the world are studying new ways to use immunotherapy to treat cancer. Some of these are discussed here.
Newer monoclonal antibodies
Monoclonal antibodies (mAbs) have already become an important part of the treatment for many cancers. As researchers have learned more about what makes cancer cells different from normal cells, they have developed mAbs to exploit these differences. They have also developed newer forms of mAbs, attaching them to drugs or other substances to make them more powerful. New mAbs are now being studied for use against many types of cancer. A few are listed here.
Breast cancer
A conjugated mAb known as trastuzumab-DM1 (or T-DM1) combines the trastuzumab (Herceptin) antibody, which targets the HER2/neu protein, with a chemo drug. It has shown promise in early studies of women whose breast cancer no longer responds to trastuzumab alone.
Another mAb, pertuzumab, targets a different part of the HER2/neu protein. It may be helpful when used along with trastuzumab to treat certain breast cancers.
Leukemias and lymphomas
Several newer mAbs are being studied in clinical trials for people with different types of leukemia and lymphoma.
Ovarian cancer
An mAb that attaches to certain antigens on both ovarian cancer cells and to certain spots on T cells (a bi-specific antibody) has shown promise when used with interleukin-2 (IL- 2). The antibody causes T cells to bind to and attack the cancer cells. Early studies have shown that radiolabeled mAbs against ovarian cancer may help some women live longer. Bevacizumab (Avastin), another mAb, slows the growth of tumor blood vessels by targeting the VEGF protein. It can slow the growth of advanced ovarian cancer, although it’s not yet clear if it helps women live longer.
Newer cancer vaccines
Vaccines are not yet considered a major treatment for cancer. But there are many different types of vaccines now being studied to treat a variety of cancers.
Breast cancer
Early studies have found that autologous vaccine therapy may lengthen the remission and survival times of some women with early breast cancer. This approach is being studied further.
A HER2/neu peptide (a small part of the HER2/neu protein), used as the antigen in a vaccine, has been shown to cause an increased immune response against the HER2/neu receptor on cancer cells. It is being studied further. Other specific antigen vaccines are also promising. These vaccines are almost always used after primary therapy (lumpectomy and radiation therapy, or mastectomy) and sometimes together with hormonal therapy or chemotherapy, to try to keep the cancer from coming back.
Cervical cancer
While HPV vaccines already available may help prevent some of these cancers, other HPV vaccines that may help treat this cancer are now being tested in clinical trials. These vaccines try to cause an immune reaction to the parts of the virus that aid the growth of cervical cancer cells. This may kill the cancer cells or stop them from growing.
Colorectal cancer
A number of autologous and allogeneic tumor cell vaccines have shown early promise in treating colorectal cancer, but so far none have been shown to lengthen survival time. Some vaccines against the carcinoembryonic antigen (CEA) protein have improved the immune response in a large portion of colorectal cancer patients, but the studies have not been going on long enough to see whether this lengthens remission or survival times.
Kidney cancer
Whole tumor cell vaccines given along with the adjuvant BCG have shrunk tumors in a small number of people with advanced kidney cancer in early studies. Researchers are also studying DNA vaccines that insert genes (segments of DNA) into cancer cells, causing the cells to make cytokines. These cytokines help the immune system recognize the cancer cells and also help activate immune system cells to attack those cells.
Lymphoma
Several vaccines have shown promising results in early clinical trials against B-cell non- Hodgkin lymphomas, but they are not yet US Food and Drug Administration (FDA)- approved.
Lung cancer
Stimuvax® (BLP25) is a peptide vaccine that is encased in a fat droplet (liposome) to make it work better. A small study of patients with advanced non-small cell lung cancer suggested it might improve survival time. Larger studies are being done to try to confirm this.
Melanoma
Although no melanoma vaccines are FDA-approved yet, recent studies have found that some autologous and allogeneic tumor cell vaccines, as well as antigen vaccines, have shrunk tumors and helped some patients live longer. Dendritic cell vaccines have also been shown to shrink tumors in some patients. Some newer studies combine vaccines with IL-2 or newer adjuvants to further stimulate the immune reaction. There is a lot of research going on in this area.
Pancreas cancer
GVAX is a tumor cell vaccine. It is made by modifying pancreatic cancer cells in the lab to express GM-CSF (to help stimulate the immune system). The cells are irradiated so they can’t grow any more. They are then injected into the patient to cause an immune response. In a small early study, patients who got the vaccine combined with the mAb ipilimumab (Yervoy®), which boosts the immune system, lived longer than expected. This vaccine is now being looked at in larger studies.
Prostate cancer
Many prostate cancer vaccines are designed to cause immune responses to antigens found only on prostate cells, such as prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSMA).
A version of the GVAX vaccine using prostate cancer cells has shown some promise in early studies. This vaccine is now being tested in larger studies of prostate cancer. Another prostate cancer vaccine (PROSTVAC-VF) uses a virus that has been genetically modified to contain PSA. The patient’s immune system should respond to the virus and begin to recognize and destroy cancer cells containing PSA. Early results with this vaccine have been promising.
Other ways to boost the immune system
Some other forms of immunotherapy now being studied try to boost specific parts of the
immune system. These types of treatments have shown promise, but they are complex
and so far are available only through clinical trials being done at major medical centers.
Lymphokine-activated killer cell therapy
Scientists can make large numbers of active, cancer-fighting T cells in the lab by treating a small number of a patient’s T cells in a test tube with the cytokine interleukin-2 (IL-2). After being returned to a patient’s bloodstream, these special cells, now called lymphokine-activated killer cells (or LAK cells), are more effective against cancer cells. Researchers are now testing several ways to use these very active cancer-fighting cells. LAK cell therapy has shown promising results in animal studies, where it shrunk tumors in animals with lung, liver, and other cancers. Although clinical trials in humans have not yet been as successful, researchers are constantly improving LAK cell techniques. They are testing these newly improved methods against melanoma, brain tumors, and other cancers.
Tumor-infiltrating lymphocyte vaccine with interleukin-2
Researchers have found immune system cells deep inside some tumors and have named these cells tumor-infiltrating lymphocytes (TILs). These cells can be removed from tumor samples taken from patients and made to multiply in the lab by treating them with IL-2. When injected back into the patient, these cells can be active cancer fighters. Treatments using TILs are being tested in clinical trials in people with melanoma, kidney cancer, ovarian, and other cancers. Early studies of this approach by researchers from the National Cancer Institute have been promising, but its use may be limited because doctors may not be able to get TILs from all patients.
To learn more More information from your American Cancer Society
We have some related information that may also be helpful to you. You can find these on our Web site or order them from our toll-free number, 1-800-227-2345.
Targeted Therapy Oncogenes and Tumor Suppressor Genes Clinical Trials: What You Need to Know Questions People Ask About Cancer (also available in Spanish)
Understanding Chemotherapy: A Guide for Patients and Families (also available in Spanish)
National organizations and Web sites*
Along with the American Cancer Society, other sources of information and support include:
National Cancer Institute
Toll-free number: 1-800-4-CANCER (1-800-422-6237)
Web site: www.cancer.gov
*Inclusion on this list does not imply endorsement by the American Cancer Society.
No matter who you are, we can help. Contact us anytime, day or night, for cancer-related
information and support. Call us at 1-800-227-2345 or visit www.cancer.org.
